Global Regulatory Affairs Outsourcing Market – Snapshot
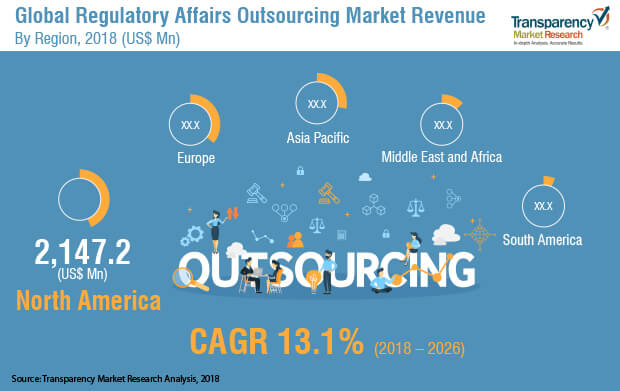
According to a new market report published by Transparency Market Research titled “Regulatory Affairs Outsourcing Market – Global Industry Analysis, Size, Share, Growth, Trends, and Forecast, 2018–2026,” the global regulatory affairs outsourcing market is expected to reach US$ 12,806.9 Mn by 2026, expanding at a CAGR of 13.1% from 2018 to 2026. North America held a prominent share of the regulatory affairs outsourcing market in 2017 and is projected to be at the forefront of global demand. Also, the Asia Pacific regulatory affairs outsourcing market is expected to expand at a significant CAGR.
Get Sample Copy:
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=51408
Regulatory affairs departments play a vital role in the life sciences industry. With the changing regulatory environment and requirements, various companies tend to outsource regulatory affairs as it is beneficial in terms of time and money. Though large pharmaceutical companies have their in-house regulatory affairs department, managing large regulatory departments can be very expensive and the scope of knowledge and expertise required to manage these regulatory departments is limited. Regulatory affairs and operations encompass a wide range of processes such as country-specific regulatory affairs, report publishing, submission planning, regulatory data management, regulatory strategy, dossier conversion, literature searches, and others. In addition, expensive clinical trials and demand for reduced time required for commercialization of new drugs will further support the growth of the regulatory affairs outsourcing market.
Regulatory consulting and legal representation segment to expand rapidly during the forecast period
The regulatory affairs outsourcing market can be segmented based on services and geography. Based on services, the market has been segmented into regulatory submissions; clinical trial applications and product registrations; regulatory writing and publishing; regulatory consulting and legal representation; and others. The others segment includes maintenance and post-marketing surveillance. Regulatory writing and publishing segment holds the largest market share. This is attributed to the increased frequency of outsourcing medical writing, clinical trial summaries, drafting of informed consent forms and other services. Also, regulatory consulting and legal representation segment is expected to expand at the highest CAGR during the forecast period. This is due to the increasing need for the consultation specifically with respect to country wise filing strategies and for gap analysis.
Grab an exclusive PDF Brochure of this report:
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=51408
North America is expected to hold a substantial share in the overall market
Based on geography, the global regulatory affairs outsourcing market has been divided into North America, South America, Europe, Asia Pacific, and Middle East & Africa. North America is expected to hold a prominent share and account for more than 40% of the global market in 2026, followed by Europe. The growth in the North America market is because majority of the large contract research organizations (CROs) are domiciled in this region. The U.S. regulatory affairs outsourcing market accounted for the largest share compared to the Canada market due to implementation of well-structured regulatory policies along with huge presence of leading pharmaceutical companies. As a part of the strategy, several service providers are investing in tools development specific to a particular application and are focused on new product developments, partnerships, and mergers and acquisitions in order to increase geographical reach in the global regulatory affairs outsourcing market.
Request For Customization:
https://www.transparencymarketresearch.com/sample/sample.php?flag=CR&rep_id=51408
Major players operating in the global regulatory affairs outsourcing market include PAREXEL International Corporation, WuXi AppTec, IQVIA Holdings Inc., Accell Clinical Research LLC, Clinilabs Inc., Criterium, Inc., Pharmaceutical Product Development, (PPD) LLC, Promedica International, Charles River Laboratories International, Inc., ICON plc., BlueReg Group, Covance, Inc., Dr. Regenold GmbH, FMD K&L, Genpact Limited, ProductLife Group S.A., Intertek Group plc,Kinapse Ltd, Medpace, Inc., Navitas Life Sciences, PharmaLex GmbH, PRA Health Sciences, Inc., Syneos Health, Inc., and Verisk 3E.
Read Our Latest Press Release:
About Us
Transparency Market Research is a next-generation market intelligence provider, offering fact-based solutions to business leaders, consultants, and strategy professionals.
Our reports are single-point solutions for businesses to grow, evolve, and mature. Our real-time data collection methods along with ability to track more than one million high growth niche products are aligned with your aims. The detailed and proprietary statistical models used by our analysts offer insights for making right decision in the shortest span of time. For organizations that require specific but comprehensive information we offer customized solutions through ad-hoc reports. These requests are delivered with the perfect combination of right sense of fact-oriented problem solving methodologies and leveraging existing data repositories.
Contact
Transparency Market Research State Tower,
90 State Street,
Suite 700,
Albany NY – 12207
United States
USA – Canada Toll Free: 866-552-3453