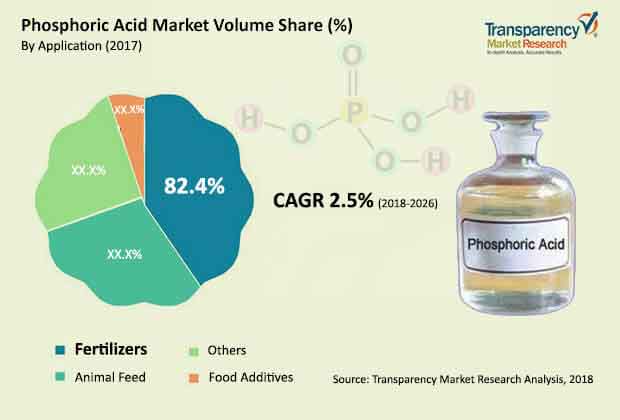
Phosphoric Acid Market – Snapshot
Phosphoric acid, also known as orthophosphoric acid, is a mineral and weak acid with chemical formula H3PO4. In terms of production value, phosphoric acid is the inorganic acid produced and consumed in the highest amount. In terms of volume, it is the second-most produced and consumed inorganic acid, after sulfuric acid. The leading use of phosphoric acid is in the manufacture of phosphate chemicals. Phosphoric acid can be produced by two commercial methods: wet process and thermal process. Wet process phosphoric acid is used in fertilizer production. Thermal process phosphoric acid is of higher purity and it is used in the manufacture of high-grade chemicals, pharmaceuticals, detergents, food products, beverages, and other non-fertilizer products.
In a wet process facility, phosphoric acid is produced by reacting sulfuric acid (H2SO4) with the naturally occurring phosphate rock. The phosphate rock is dried, crushed, and then continuously fed into the reactor along with sulfuric acid. The reaction combines calcium from the phosphate rock with sulfate, forming calcium sulfate (CaSO4) and phosphoric acid.
Request Brochure @
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=2435
The thermal process requires raw materials such as elemental (yellow) phosphorus, air, and water for the production of phosphoric acid. Following steps are used in the thermal process: combustion, hydration, and demisting. In combustion, liquid elemental phosphorus is burned (oxidized) in ambient air in a combustion chamber at temperatures ranging from 1650°C to 2760°C (from 3000°F to 5000°F) to form phosphorus pentoxide. Phosphorus pentoxide is then hydrated with dilute phosphoric acid or water to produce strong phosphoric acid in liquid form. Demisting removes the phosphoric acid mist from the combustion gas stream before releasing it into the atmosphere.
The demand for phosphoric acid is primarily driven by the increasing demand for the production of phosphate fertilizers, which is closely related to the growing population worldwide. According to the UN Department of Economics & Social Affairs, the global population is expected to increase by two billion and reach 9.7 billion by 2050. The increasing population propels the demand for food, which is fulfilled by crops nourished with fertilizers. Developing countries and regions are using fertilizers at a much higher rate than developed countries. Phosphorus is an essential plant nutrient. It is uptaken by plant roots, usually as a dihydrogen phosphate ion (H2PO4-) derived from phosphoric acid (H3PO4).
REQUEST FOR COVID19 IMPACT ANALYSIS –
https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=2435
Fertilizers are used to compensate phosphorus deficiencies in the soil. Diammonium phosphate (DAP), monoammonium phosphate (MAP), and trisodium phosphate (TSP) are three important fertilizers derived from phosphoric acid, which are used across the globe to increase the crop yield and satisfy the increasing demand for food. Diammonium phosphate fertilizers are rich in mineral phosphorus. They are widely used in the production of grain and row crops (such as corn, wheat, rye) and some lawn grasses. Thus, increase in the demand for phosphate fertilizers boosts the demand for phosphoric acid.
The environmental impact of phosphoric acid and phosphates in general has been investigated over the years and with the recent and relatively clear results, regulations have been imposed on the use of these chemicals. Phosphates and phosphoric acid affect water systems and pollute the water. High usage of phosphates in water bodies leads to an imbalanced water system. Phosphates provide nutrients to plants in a water body such as floating algae, which results in their abrupt growth. This decreases the supply of oxygen to animals in the water body. The entire cycle causes an imbalance, harming the human life, as humans rely on water bodies for sustenance. This cycle of pollution is a well-documented fact and is also the reason why phosphoric acid are being heavily regulated and banned in several regions across the world. Thus, the environmental impact of phosphates hampers the market for phosphoric acid.
Due to the rising demand for food, quality of crops needs to be improved and crop production needs to be increased. This is achieved by using phosphate fertilizers on crops. This, in turn, fulfills the global food demand. Market players, along with government bodies and cooperatives, are adopting various measures to improve awareness among farmers. These initiatives include educating farmers regarding the use of online portals developed specifically to provide information related to phosphate products. Detailed information is provided and frequently updated studies on availability, effectiveness, correct time, and amount of application of phosphate fertilizers are uploaded on these portals. Seminars are organized in collaboration with farmer cooperatives and government bodies to reach out to farmers with the appropriate information. Major market players have launched interactive online portals with live assistance by their regional representatives to keep farmers updated and resolve their plant-disease-related queries.
Key players operating in the global phosphoric acid market are focusing on capacity expansion and mergers, which is likely to propel the market during the forecast period. The market players of the phosphoric acid hold almost half of the total global phosphoric acid capacity and are giving fertilizers based products derived from the phosphoric acid.
Phosphoric Acid Market to Gain Significant Momentum Owing to Increasing Demand from Agricultural Sector
The high nutrient content in phosphoric acid helps increase its demand n the agricultural industry and is considered a key factor aiding in expansion of the global phosphoric acid market. Phosphoric corrosive or phosphoric acid is also known as orthophosphoric corrosive. It is a mineral and frail corrosive with compound recipe H3PO4. As far as creation esteem, phosphoric corrosive is the inorganic corrosive delivered and devoured in the most elevated sum. Regarding volume, it is the second-most created and burned-through inorganic corrosive, after sulfuric corrosive.
The main utilization of phosphoric corrosive is in the production of phosphate synthetic compounds. Phosphoric corrosive can be created by two business techniques: wet cycle and warm cycle. Wet cycle phosphoric corrosive is utilized in manure creation. Warm cycle phosphoric corrosive is of higher immaculateness and it is utilized in the assembling of high-grade synthetic substances, drugs, cleansers, food items, drinks, and other non-manure items.
The interest for phosphoric corrosive is fundamentally determined by the expanding interest for the creation of phosphate composts, which is firmly identified with the developing populace around the world. Owing to the rising interest for food, nature of yields should be improved and crop creation should be expanded. This is accomplished by utilizing phosphate composts on yields. This, thusly, satisfies the worldwide food interest.
Market players, coupled with the government bodies and cooperatives, are embracing different measures to improve mindfulness among ranchers. These activities incorporate teaching ranchers with respect to the utilization of online gateways grew explicitly to give data identified with phosphate items. Definite data is given and oftentimes refreshed investigations on accessibility, viability, right time, and measure of use of phosphate composts are transferred on these entries. Classes are coordinated as a team with rancher cooperatives and government bodies to contact ranchers with the proper data. Significant market players have dispatched intelligent online entrances with live help by their territorial agents to keep ranchers refreshed and resolve their plant-infection related inquiries.