Do you want to learn how to find density with mass and volume? Are you looking for the density formula and step by step explanation with examples? You can’t have chosen better. Here is a complete guide, thanks to which you will finally find density with mass and volume without any problems.
We know that math is a nightmare for some of you. But we want to prove to you that it doesn’t have to be anymore. You are 3 steps away to understand how to find density with mass and volume. First, you need to know the density formula. Second, you need to know how to use it. Third, you need to see the formula in practice. And we will guide you through all these 3 steps. Let’s start!
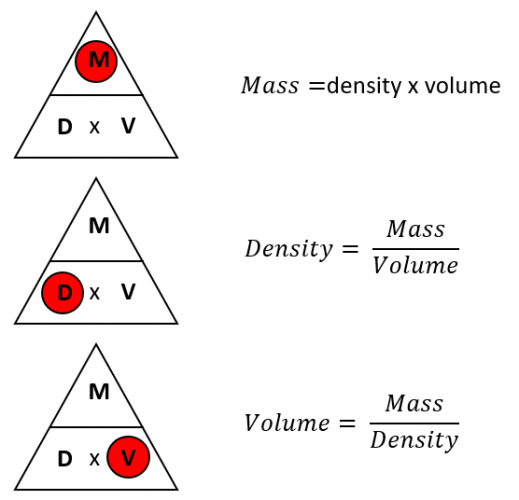
Formula to find density with mass and volume
First step is to know the formula to find density with mass and volume. So how does the density formula look? You can see it down below:
Density = Mass / Volume
It doesn’t look complicated, don’t you think? Just one simple mathematical operation to get the result. To be more precise, you need to divide the number of the mass by the number of the volume. That’s it.
You know the density formula and know how to use it, so 2 steps are behind you. Only one step is left to understand how to find density with mass and volume on your own. So let’s move on to the third step, that is to the practice.
Calculate density with mass and volume step by step
We think this part of the article will be the most interesting for you. The practice is the best way to see how something works, after all. So, move on to the examples.
The first example will be the simpler one. For instance, find density with mass and volume for the data given below:
- Mass = 5 kg;
- Volume = 2 kg/m3.
If you put the data in the density formula given above, you will get the equation looking like this:
Density = 5 kg / 2 kg/m3
The calculations for this equation are as follows:
5 kg / 2 kg/m3 = 2.5 m3
So the density is equal to 2.5m3.
Does it look complicated? We don’t think so. We are sure that the examples like this all of you will be able to calculate without any problems in just a while.
Now it is time for another example. In this case the data looks as follows:
- Mass = 100 g;
- Volume = 37.05 g/cm3.
Put the data in the density formula. It looks like this:
Density = 100 g / 37.05 g/cm3
And the calculations are as follows:
100 g / 37.05 g/cm3 = 2.69905533 cm3
So the density is equal to 2.69905533 cm3 in this case. After rounding off to one decimal place it is 2.7 cm3. A little tidbit – it is the density of aluminium.
The calculations are not so easy now, right? If you don’t need an exact result, you can use rounded off numbers to find density with mass and volume. Then the calculations will look like this:
100 g / 37 g/cm3 = 2.7027027 cm3
Now the calculations are a little bit easier than before. And as you can see, the result is almost the same!
Do you want to check the correctness of your result? Do you have a complicated example to calculate and need an exact result? You can use the density converter – an online app which will find the density with mass and volume in just a second.
We hope you see now that math doesn’t have to be a nightmare. So it is time to make your move – follow the steps explained in the article and find density with mass and volume on your own.