Extended Text Labels Market: Introduction
Transparency Market Research delivers key insights for the extended text labels market in its published report, which include global industry analysis, size, share, growth, trends, and forecast for 2024-2025. In terms of revenue, the global extended text labels market is projected to expand about 1.3x its current market value by the end of 2025, owing to ever-increasing demand from end usage such as general pharmaceuticals, clinical trials, animal health, and others across several countries, about which TMR offers detailed insights and forecasts in the extended text labels market report.
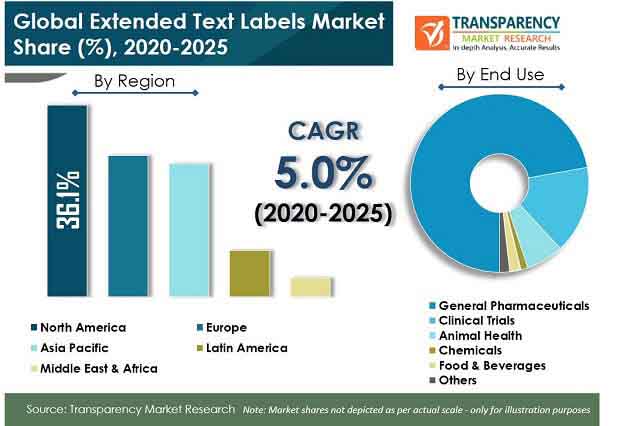
Extended text labels are used primarily in end-use industries, including pharmaceuticals, clinical trials, animal health, chemicals, and food & beverages. Extended text labels are also known as extended content labels. These are multi page labels, which provide enough printing space on limited printing space of packaging products. These labels are predominantly used in pharmaceuticals and clinical trials industries. TMR segmented the analysis of extended text labels market based on various factors such as label type, product type, material type, application, and end use across five regions. As per the TMR analysis, booklet/leaflet type extended text labels are expected to hold the largest market share during the forecast period, as they offer brand owners a modern, stylish way to market their products to end users.
For More Details, Request A Sample Report@ https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=18644
Regulatory Environment Associated to Labeling of Pharmaceutical Products to Drive Extended Text Labels Market
Many countries around the world are increasingly implementing strict laws and regulations related to labeling in pharmaceutical and clinical trials products. Recent trend shows manufacturers in prominent end-use industries are increasingly adopting extended text labels to be in compliance with governments’ regulations. For instance, the U.S. FDA issued new regulations for label requirements with expanded information. Such detailed labeling aims to provide information to the drug prescriber or doctor more than to the consumer.
Besides this, The European Medicines Agency (EMA) publishes information on excipients for the inclusion in more detailed labelling of human medicines. An excipient is a constituent of a medicine other than the active substance, added in the formulation for a specific purpose. Recent trends in packaging shows that packaging sizes are continuously decreasing, but the information printed on labels is increasing. This challenge can only be tackled with extended content labels.
Rising Demand for Clinical Trials Due to Increase in Number of New Drug Discoveries and Formulations
Pharmaceutical manufacturers are continuously looking for new drugs and for this purpose, they are heavily investing in research & development. The fierce competition in the pharmaceutical industry is also pushing manufacturers to heavily invest in research & development for new or updated drugs or formulations. The increase in new drug discoveries and formulations is pushing the demand for clinical trials globally. Major countries where clinical trials industry has lucrative opportunity are India and China that has a large growth potential in Asia Pacific; this industry in Brazil has a large growth potential in Latin America. The growth of the Eastern Europe clinical trials industry is driven by Poland, Hungary, and Russia.