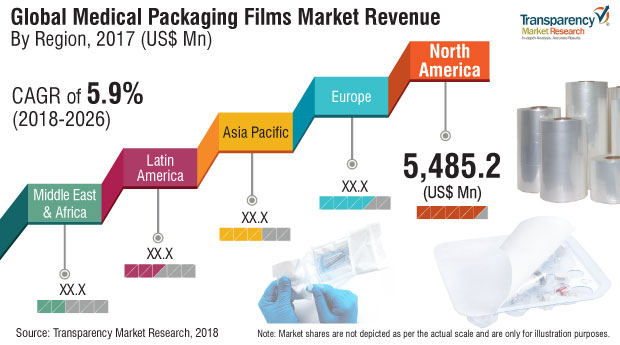
The global medical packaging films market was valued at US$ 14,092 million in 2017. The market is forecast to expand at a CAGR of 5.9% during the forecast period, 2018-2026.
Medical packaging films market primarily consists of high barrier films, co-extruded films, and formable films. Medical packaging films provide a high barrier against moisture, water, oxygen, and other gases. Medical packaging films are used in the manufacture of packaging formats such as bags & pouches, blister packs, labels, sachets, and wraps. Materials used to manufacture medical packaging films include, plastic, aluminum, and oxides. High barrier films segment is further segmented into metallized and coated films. Metallized medical packaging films segment is expected to witness a positive growth, registering a CAGR of 5.1%. Coated medical packaging films are coated with polymers or aluminum to improve their barrier property. Medical packaging films are used as lidding on trays. For tray lids, the seal area should transmit the peeling force smoothly around the package. The shape of the seal and design and location of the peel tabs affect the relative ease of opening. Bags & pouches are used for the packaging of gloves, catheters, syringes, dressings, bandages, etc. Medical packaging films tend to provide less physical protection than the rigid ones. The sealing of medical device packaging is critical. Inappropriate sealing can negatively affect the package integrity of the medical packaging films.
Request for a sample:
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=28790
Manufacturers are exploring the clinical requirement for two sterile barriers on the medical device. Depending on the attributes of the device, including its size, design specifications, complexity, number of components, and the clinical procedure itself, having two sterile barriers in medical packaging films is an unnecessary use of packaging materials and resources. It is estimated that by the year 2050, over 5% of the U.S. population will be over the age of 85 and will overall drive the medical packaging films market. The aging trend is not limited to the United States but is a global phenomenon that warrants the consideration of packaging for all types of products. Not only do aging consumers use medical products at a higher per capita rate than the general population, but they are also at risk of inappropriate product use for a variety of reasons. Therefore, manufacturers of medical packaging films have engaged in patient-centric packaging.
Medical packaging films need to be approved for use with a specific medical device by the Food & Drug Administration. However, generally, the FDA regulates medical devices but leaves the choice of acceptable medical packaging films largely up to the device makers. The FDA doesn’t approve medical packaging films as independent products. Medical packaging films are validated for medical use by the device maker. The validation specifies and essentially freezes every aspect of production, including exact resin grades and additives, extrusion parameters, and even which specific extruder in a plant makes the medical packaging films. This relatively inflexible system means that a medical packaging films processor must be assured of a stable, long-term supply of approved resins. If a resin company stops producing one of those materials, medical packaging films have to be requalified with new resin.
North America has shown impressive growth in medical packaging films production as well as consumption, such that it towers over the rest of the countries in the APAC region, and beyond. The Chinese medical packaging films market currently accounts for more than 60% of the global medical packaging films consumption alone. North America’s medical packaging films market was significantly boosted, owing to the increase in geriatric population and is expected to continue the momentum of growth of medical packaging films market during the forecast period. Therefore, medical packaging films manufacturers are expected to eye the Asia Pacific region for potential business expansion. Therefore, the outlook for the global medical packaging films market is expected to be positive during the forecast period.
Patient-centric Packaging:
Blister strips using formed aluminum foil or laminate for the base layer usually take up a bigger footprint than those made from medical packaging films, because of the limitations in the depth and gradient of pocket size that can be achieved with this material. Larger packs are not favored by patients or pharmacists, so for products requiring a high barrier to moisture, producers should consider the use of high-barrier medical packaging films instead, as these materials allow the production of smaller footprint blisters whilst still providing high moisture barrier properties. The trend for calendared blister medical packaging films is witnessed in regions such as North America and Europe and is soon to gain a market footprint in the APAC and MEA region.
Ask for brochure:
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=28790
Market Taxonomy:
The global medical packaging films market has been segmented on the basis of material type, product type, application, end-use, and region.
The various material types considered in the medical packaging films market study include plastic, aluminum, and oxides. Of these, the plastic medical packaging films segment accounts for the lion’s share of the global medical packaging films market.
On the basis of product type, the global medical packaging film market has been segmented into high barrier films, co-extruded films, and formable medical packaging films. The high barrier segment has been further subdivided into two categories, which include metallized and coated medical packaging films. The metallized films segment is expected to heavily dominate during the forecast period, accounting for more than 70% of the metallized medical packaging films demand.
Read TMR Research Methodology at: https://www.transparencymarketresearch.com/methodology.html
Competitive Landscape:
Key players considered in the report on medical packaging films market include 3M Company, Winpak Ltd, Bemis Company, Inc., Dunmore Corporation, Berry Global Group, Inc., Honeywell International, Toray Plastics (America), Inc., Klöckner Pentaplast Europe GmbH & Co. KG, Glenroy Inc., and Renolit Group.
Read Our Latest Press Release:
- https://www.prnewswire.com/news-releases/affordability-and-beneficial-properties-to-serve-as-vital-growth-factors-for-construction-tape-market-during-forecast-period-of-2020-2030-tmr-301221294.html
- https://www.prnewswire.com/news-releases/global-higher-education-solutions-market-to-thrive-on-growing-popularity-of-cloud-computing-and-high-consumption-of-digital-content-tmr-301219732.html
About Us
Transparency Market Research is a next-generation market intelligence provider, offering fact-based solutions to business leaders, consultants, and strategy professionals.
Our reports are single-point solutions for businesses to grow, evolve, and mature. Our real-time data collection methods along with ability to track more than one million high growth niche products are aligned with your aims. The detailed and proprietary statistical models used by our analysts offer insights for making right decision in the shortest span of time. For organizations that require specific but comprehensive information we offer customized solutions through ad-hoc reports. These requests are delivered with the perfect combination of right sense of fact-oriented problem solving methodologies and leveraging existing data repositories.
Contact
Transparency Market Research State Tower,
90 State Street,
Suite 700,
Albany NY – 12207
United States
USA – Canada Toll Free: 866-552-3453
Email: [email protected]





