D-dimer Testing Market: Overview
At present, the in vitro diagnostics market is one of the fastest growing markets globally. This remarkable growth can be attributed to the improved health care awareness, preference of the people for preventive health checkups, accessibility to disease specific tests, and shift from manual to semi-automated and automated instruments. Diagnostic laboratories are facing challenges in delivering high-quality, efficient and timely testing solutions due to rising health care awareness and stringent regulations that put pressure on the health care budgets. This has led to growing adoption of automated solutions and new analyzers that deliver high throughput in lesser time and with enhanced efficiency. Point-of care testing has gained momentum in the past few years to meet the demand for quick turnaround time, thus accelerating reliable results. D-dimer assays and POC kits have been developed to confirm the exclusion of deep vein thrombosis (DVT), pulmonary embolism (PE), and disseminated intravascular coagulation (DIC).
Request Brochure for Report –
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=2398
D-dimer Testing Market: Snapshot
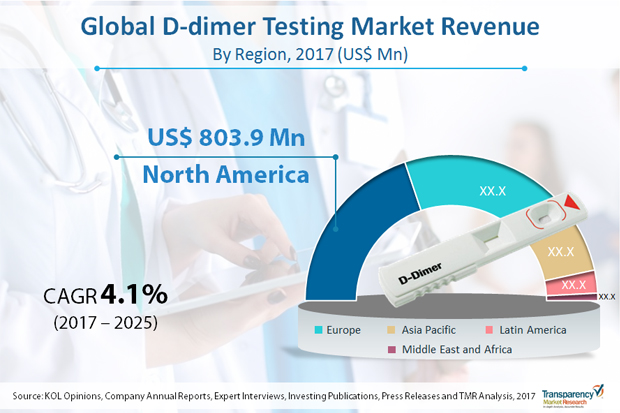
Blood tests that are used to measure the blood ability and time to clot are known as coagulation testing for the diagnosis of hemostasis system. Clotting disorders can cause excessive bleeding or clotting and are fatal. D-dimer testing is ordered if the physician suspects clotting disorders. There are number of bleeding disorders which are either acquired or inherited. The most common disorders are deep vein thrombosis (DVT), blood factor deficiency etc.The global market for D-dimer testing was worth US$ 1,966 million in 2016 and is anticipated to register CAGR of over 4.0% from 2017 to 2025, with deep vein thrombosis application dominating the overall global market. Advancements in D-dimer testing technology has led to development of wide range of products that have enabled clinicians to provide proper emergency assistance to patients. The concept of automation has extended to D-dimer testing as well, which is less labor intensive, rapid, and easy-to-use. Combination of various technologies has increased the yield and productivity. This technological revolution in D-dimer testing is expected to drive the market from 2017 to 2025. Prevalence of numerous diseases such as cancer, diabetes, and neurological disorders has increased due to rise in geriatric population and changing lifestyle in developing economies. For instance, data released by the WHO suggested that the global geriatric population is expected to increase from 524 million in 2010 to 1.5 billion by 2050.
Request for Analysis of COVID19 Impact on D-dimer Testing Market –
https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=2398
North America to Capture Major Share of Global D-dimer Testing Market
Geographically, North America accounts for the largest market share in terms of revenue in 2016 and is expected to maintain its position by the end of forecast period. Europe is followed by North America in term of number of D-dimer tests performed across the globe. Increasing research activities to improve specificity of D-dimer tests in order to identify the presence of any coagulation diseases such as DVT, PE and DIC are the major drivers of the D-dimer market in Europe. According to the Society of Interventional Radiology (U.S.), about 600,000 new cases of DVT are diagnosed in the U.S. each year. An increase in the number of laboratories and growing demand for D-dimer POC tests, development of public health care systems, rising interest of big pharmaceutical players, and increasing population, are several factors expected to drive the D-dimer testing market in Asia-Pacific, Latin America and MENA regions.
Key Players Operating in Global D-dimer Testing Market
The key players contributing to the global D-dimer testing market include Abbott Laboratories, Beckman Coulter, Inc., Bio/Data Corporation, Becton, Dickinson and Company, Grifols, S.A., F. Hoffman-La Roche Ltd., Helena Biosciences, Siemens Healthcare, Sysmex Corporation, and Thermo Fisher Scientific The future holds challenges for the market leaders and niche suppliers due to continuous expansion through acquisitions and growing number of customers. Major technological trend observed among the leading players operating in the global D-dimer testing market is the introduction of next-generation integrated blood analyzer. For instance, On October 31, 2017, Abbott announced the FDA 510(k) clearance for Alinity ci-series.
Buy D-dimer Testing Market Report:
https://www.transparencymarketresearch.com/checkout.php?rep_id=2398<ype=S
About Us
Transparency Market Research is a global market intelligence company providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for several decision makers. Our experienced team of analysts, researchers, and consultants use proprietary data sources and various tools and techniques to gather and analyze information.
Our data repository is continuously updated and revised by a team of research experts so that it always reflects latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact
Transparency Market Research,
90 State Street, Suite 700,
Albany, NY 12207
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com/





