Chemotherapy and/or radiotherapy is used for the treatment of most cancers. However, chemotherapy and radiotherapy have a very common side effect called neutropenia, and pegfilgrastim is required for its treatment.
A study conducted on patients who received chemotherapy in 2016-2017 to evaluate the occurrence of chemotherapy-induced febrile neutropenia (CIFN) showed that, out of 200 patients, 9.5% patients developed neutropenia.
Request for Sample https://www.factmr.com/connectus/sample?flag=S&rep_id=1494
Neutropenia patients are highly susceptible to infections, and for this reason, it is a life-threatening disease. Pegfilgrastim is advantageous over filgrastim in relatively decreasing the incidence of febrile neutropenia. In the future, pegfilgrastim biosimilars will provide better access to this treatment.
The pricing of pegfilgrastim biosimilar is around 25% to 30% lower as compared to originator products (i.e. Neulasta by Amgen). Lower prices of pegfilgrastim biosimilars are expected to enhance their adoption for neutropenia treatment in developed as well as developing countries, as multiple regional and global players are seeking to get marketing approval in various countries.
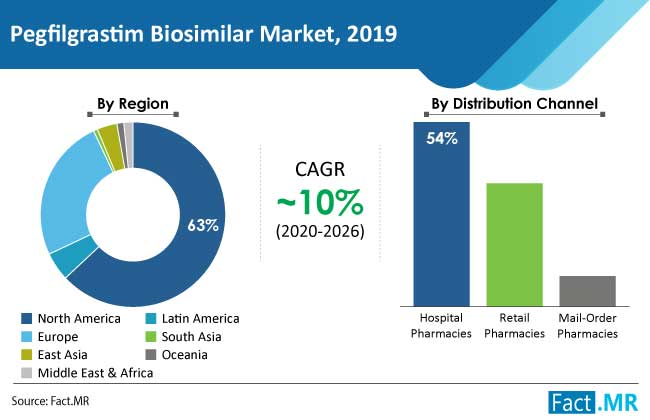
All in all, rising incidence of cancer and increasing use of chemotherapy for treatment will aid the expansion of the pegfilgrastim biosimilar market, which was valued at US$ 944 Mn in 2019, and is expected to exhibit a CAGR of over 10% over the forecast period (2020–2026).
Key Takeaways from Pegfilgrastim Biosimilar Market Study
- The hospital pharmacies segment under the distribution channel category held half of the global pegfilgrastim biosimilar market share in 2019, owing to increasing cancer treatments such as chemotherapy and others. Retail pharmacies followed due to increasing number of prescriptions.
- North America holds almost 3/4 of the global pegfilgrastim biosimilar market, followed by Europe, owing to large number of product launches in these regions.
- The East Asia market year-on-year growth is expected to rapidly surge in the near future. This growth is due to increasing pool of patients and advancements in healthcare with government support, which will propel pegfilgrastim biosimilar market growth in Asian countries.
- The COVID-19 pandemic that has swept the world is projected to have only a moderate impact on the progress of the pegfilgrastim biosimilar market.
Request for Methodology https://www.factmr.com/connectus/sample?flag=RM&rep_id=1494
“Increasing demand for cost-effective therapeutics such as biosimilars and patent expiration of blockbuster biologics will provide competitive benefits to market players in terms of new product development,” says a Fact.MR analyst.
Increasing Consolidation among Key Players and New Product Launches
Increase in value chain partnerships and market consolidation activities such as collaborations, partnerships, and M&A among market players will be a significant tool to increase their capabilities and speed to market for new launches.
For instance, Mylan and Biocon collaboratively worked on a partnership to launch Pegfilgrastim biosimilar Fulphila.
Manufacturers are making efforts to develop biosimilars, and, so far, in 2024, there were 4 biosimilar launches in the United States, and many more are anticipated to be launched. It is estimated that, the pipeline from 2024 to 2025 will have at least 5 approved biosimilars.
Looking for More Information?
The research study on the pegfilgrastim biosimilar market by Fact.MR incorporates an unbiased assessment of key demand-driving factors and trends, which have shaped the landscape of the pegfilgrastim biosimilar market over 2018–2019, and includes a detailed assessment of key parameters that are expected to exert influence over 2024–2026.
For in-depth competitive analysis, buy now https://www.factmr.com/checkout/1494/S
Market statistics have been presented based on distribution channel (hospital pharmacies, retail pharmacies, and mail-order pharmacies), across seven major regions.
About Fact.MR
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions. While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise. Spanning a wide range – from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed. Our sales offices in United States and Dublin, Ireland. Headquarter based in Dubai, UAE. Reach out to us with your goals, and we’ll be an able research partner.
Contact:
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E: sales@factmr.com
Corporate Headquarter:
Unit No: AU-01-H Gold Tower (AU),
Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers,
Dubai, United Arab Emirates





